
Category

Portable a Scan P Scan Ultrasoud Biometer/Pachymeter for Ophthalmology with Touch Screen (MD
MD-1000A/P Ultrasonic Biometer for Ophthalmology Specifications Models MD-1000A/P A-Biometer/Pachymeter MD-1000A A-Biome
Basic Info.
| Model NO. | MD-1000A/P |
| Trademark | MEDA |
| Origin | China |
| HS Code | 90185000 |
| Production Capacity | 300 Sets/Year |
Product Description
MD-1000A/P Ultrasonic Biometer for Ophthalmology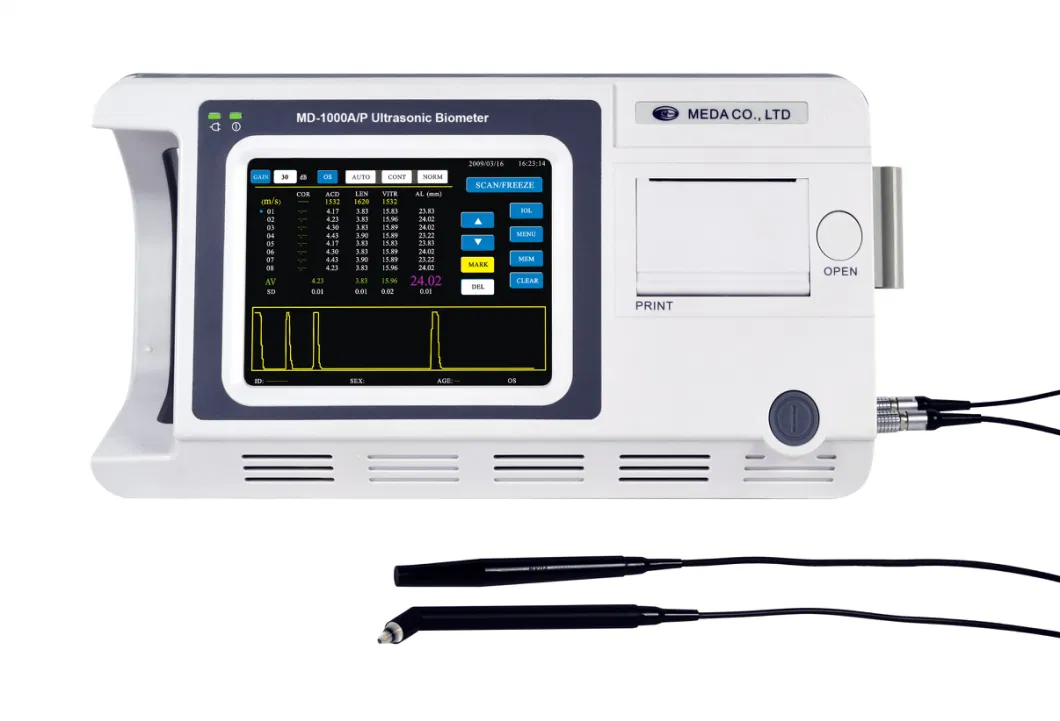
Specifications
| A-Scan (MD-l000A & MD-l000AP) | |
| Probe: | 10MHz with Fixation Red Light |
| Total Gain: | 100dB with an adjustable range of 0-50dB |
| Biometry Accuracy: | ±0.05mm |
| Resolution: | 0.01mm |
| Measuring Range: | 15-40mm |
| Measuring Mode: | Contact or Immersion |
| Measuring Parameters: | Anterior Chamber Depth, Lens Thickness, Vitreous Length and Axial Length |
| Measuring Modes: | Automatic (Normal, Cataract, Aphakic and Special, and Manual |
| 8 Groups of Readings with Averaging & Standard Deviation | |
| IOL Calculation | |
| General: | SRK-II, SRK-T, BINK-II, HOLLADAY, HOFFER-Q and HAIGIS |
| Post-Refractive: | History-derived, Refraction-derived, Double K/SRK-T, ROSA, SHAMMAS |
| Pachymeter (MD-1000P & MD-1000AP) | |
| Probe Frequency: | 15-20MHz |
| Display Resolution: | 1μm |
| Biometry Accuracy: | ±5μm |
| Measuring Scope: | 230-1200μm |
| Multiple Corneal Maps with Graphical Display | |
| Standard Configuration | |
| 10MHz A probe (MD-1000A & MD-1000AP) | |
| 20MHz P Probe (MD-1000P & MD-1000AP) | |
| Footswitch | |
| Test Object | |
| AC Adapter | |
ModelsMD-1000A/P A-Biometer/Pachymeter
MD-1000A A-Biometer
MD-1000P Pachymeter
Certificate
CFDA,CE,FDA
Options
Tolley, Immersion Shell, PC Suite








